
The top equation shows the formation and some reactions of the 4,6-O-benzylidene acetal, a commonly employed protective group. As a rule, benzaldehyde forms six-membered cyclic acetals, whereas acetone prefers to form five-membered acetals. Acetal derivatives have been prepared by acid-catalyzed reactions with benzaldehyde and acetone. A pyranose structure for D-glucose is drawn in the rose-shaded box on the left. The formation of acetal derivatives illustrates how subtle changes may alter this selectivity. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features.
Examples of four typical pyranose structures are shown below, both as Haworth projections and as the more representative chair conformers. We know that these molecules are actually puckered in a fashion we call a chair conformation. These Haworth formulas are convenient for displaying stereochemical relationships, but do not represent the true shape of the molecules. In the D-family, the alpha and beta bonds have the same orientation defined for the furanose ring (beta is up & alpha is down).

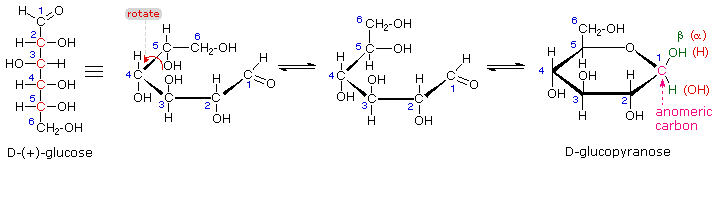
As with the furanose ring, the anomeric carbon is placed on the right with the ring oxygen to the back of the edgewise view. The cyclic pyranose forms of various monosaccharides are often drawn in a flat projection known as a Haworth formula, after the British chemist, Norman Haworth. The upper bond to this carbon is defined as beta, the lower bond then is alpha. The anomeric carbon atom (colored red here) is placed on the right. By convention for the D-family, the five-membered furanose ring is drawn in an edgewise projection with the ring oxygen positioned away from the viewer. Ribose, an important aldopentose, commonly adopts a furanose structure, as shown in the following illustration. Cyclic structures of this kind are termed furanose (five-membered) or pyranose (six-membered), reflecting the ring size relationship to the common heterocyclic compounds furan and pyran shown on the right. Five and six-membered rings are favored over other ring sizes because of their low angle and eclipsing strain. If necessary, before you attempt to study this section, review the formation of hemiacetals discussed in Section 19.10.Īs noted above, the preferred structural form of many monosaccharides may be that of a cyclic hemiacetal.


 0 kommentar(er)
0 kommentar(er)
